BRAINS IN BRIEFS
Scroll down to see new briefs about recent scientific publications by neuroscience graduate students at the University of Pennsylvania. Or search for your interests by key terms below (i.e. sleep, Alzheimer’s, autism).
Adolescent nicotine exposure fundamentally changes the brain to make subsequent morphine use in adulthood more rewarding
or technically,
Paradoxical Ventral Tegmental Area GABA Signaling Drives Enhanced Morphine Reward After Adolescent Nicotine
[See original abstract on Pubmed]
Dr. Ruthie E. Wittenberg was the lead author on this study. Ruthie is currently a postdoc in the lab of Paul Kenny at the Icahn School of Medicine at Mount Sinai. Her research is focused on the intersection of cellular immunology and addiction neuroscience. In the future, she hopes to establish an independent academic research career studying the neurobiology of substance use disorders and motivated behaviors.
or technically,
Paradoxical Ventral Tegmental Area GABA Signaling Drives Enhanced Morphine Reward After Adolescent Nicotine
[See Original Abstract on Pubmed]
Authors of the study: Ruthie E. Wittenberg, Sanghee Yun, Kechun Yang, Olivia K. Swanson, Shannon L. Wolfman, Lorianna M. Colón, Amelia J. Eisch, and John A. Dani
In the United States, drug overdose is a leading cause of death, and most drug overdoses involve the use of opioids. Opioids (sometimes called narcotics) are often medically prescribed for treating chronic pain. However, opioids, whether prescribed or not, have high abuse risk, and taking them can lead to opioid use disorder (OUD). OUD is a type of substance use disorder or addiction that involves a “problematic pattern of opioid use that causes significant impairment or distress” according to the CDC. Anyone can become addicted to opioids, but some things might make it more likely for someone to develop OUD. For example, adolescent nicotine use is a risk factor for developing OUD later in life. Most tobacco use begins during adolescence, with teens vaping or using nicotine pouches.
The link between early-life nicotine use and OUD is not well understood, so for her PhD in the labs of John Dani and Amelia Eisch, former NGG student Ruthie Wittenberg wanted to understand how nicotine use during adolescence could change the brain to promote morphine reward in adulthood.
Adolescence is the time in development when the brain is more flexible and with greater plasticity than in adulthood. One such part of the brain, the ventral tegmental area (VTA), is considered to be the start of the main reward pathway. Increased neuronal activity, altered dopamine signaling, and structural changes are examples that all contribute to increased reward sensitivity. Evidence from both human and animal studies indicate that using drugs during adolescence, when the brain is most flexible, can create long-lasting changes in the brain and in behavior that can extend into adulthood.
To understand how adolescent nicotine exposure changes reward-related behaviors in adulthood, Ruthie gave adolescent mice nicotine for 2 weeks and waited until they were older to see how they responded to opioids, like morphine. She wanted to see how adult mice who had been exposed to nicotine earlier in life responded to the rewarding properties of opioids compared to mice who had never been exposed to nicotine. Ruthie used a conditioned place preference (CPP) paradigm in mice to test reward learning. CPP works like this: there are different chambers or “contexts.” They differ in both visual and tactile cues. Each context has a different pattern of stripes or colors on the wall to help the mice be able to tell the difference between the chambers. The floor of each chamber also differs (e.g. they have distinct ridges or grooves), providing a tactile cue.
In this case, mice received morphine in one chamber, and a control drug (saline) in another chamber for 3 days. After the 3 days, Ruthie allowed the mice to move freely between the chambers and measured how much time they spent in the morphine-paired chamber compared to the saline-paired chamber. If mice spent more time in the reward chamber than the saline chamber, this indicated they were able to associate that context with the reward (the opioid), and that they remembered that this chamber felt more rewarding. Ruthie tested the mice who had been previously exposed to nicotine and mice who had never been given nicotine in the morphine CPP test, and she found that mice that had been exposed to nicotine spent more time in the morphine chamber than the group of mice that never had nicotine. The non-nicotine group still remembered the chamber where they got morphine but they spent significantly less time there than the nicotine group. In conclusion, Ruthie found that morphine was even more rewarding to the nicotine-exposed mice than the mice who were never exposed to nicotine.
Ruthie further explored what changes in the brain are happening because of adolescent nicotine use to make this behavior happen?
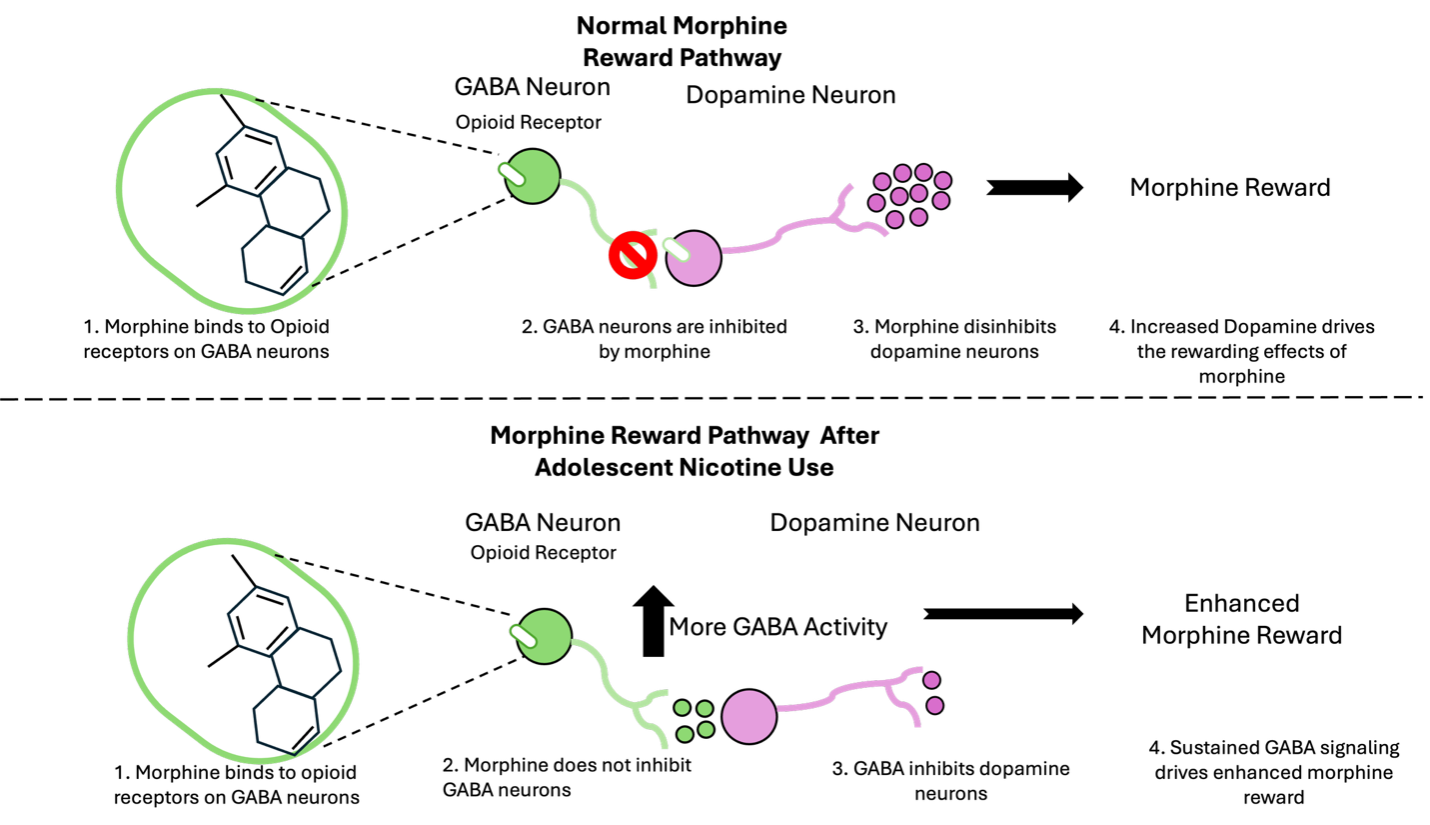
To understand the link between using nicotine in adolescence and morphine later in adulthood, Ruthie looked at the VTA, the origin of the reward pathway. The VTA is made up of neurons that release one or more neurotransmitters to communicate with each other. It is also well-known for producing dopamine, which motivates the seeking of drugs and other rewards. Additionally, the VTA releases other neurotransmitters, like GABA, which helps to quiet other neurons, like those that release dopamine. Since the brain is really flexible in adolescence, Ruthie wanted to know if changes in VTA GABA neurons could be a link between nicotine use in adolescence and morphine use in adulthood.
Chronically using nicotine in adolescence changes how VTA GABA neurons communicate with other neurons. Ruthie wanted to know how adolescent nicotine use changes how VTA GABA neurons respond to morphine in adulthood. As a result, she used patch-clamp electrophysiology to record the electrical activity of these neurons. An important thing to note is that opioids like morphine bind to endogenous opioid receptors, meaning receptors mice already have on their VTA GABA neurons. When morphine acts on these receptors, it inhibits neural activity. Moreover, GABA neurons quiet the VTA dopamine neurons and morphine quiets GABA neurons, allowing dopamine neurons to release more dopamine, leading to the rewarding effects of morphine. This process is called disinhibition, where the neurons that inhibit (GABA neurons) are inhibited themselves, which allows other neurons to start to fire.
Figure 1: Schematic representation of how the VTA reward circuit is fundamentally altered after adolescent nicotine use.
Ruthie found that adolescent nicotine use changed the way that this neural circuit was acting, such that morphine no longer disinhibits dopamine neurons (Figure 1). By recording from GABA and dopamine neurons in the VTA in adult mice, she found that among mice exposed to nicotine as adolescents, morphine no longer inhibited GABA neurons and it also did not change how dopamine neurons fired.
Ruthie’s results would suggest that sustained GABA neuron activity seemed to be driving the increased reward behavior seen in the CPP experiment. To test this, Ruthie artificially inhibited VTA GABA neurons in mice that had nicotine exposure during adolescence (enabling the VTA GABA cells of the nicotine animals to act like those of the water animals) and observed that the enhanced reward learning previously seen was blocked.
In conclusion, Ruthie determined that the VTA circuit is fundamentally changed after adolescent nicotine exposure. After adolescent nicotine use, VTA GABA neurons are no longer inhibited by morphine and additionally, mice find morphine more rewarding than normal. These findings challenge the canonical view of the brain’s reward system and advance our understanding of the mechanisms driving addiction-related behaviors. This may explain why prior drug exposure can make it more likely that people develop disorders like OUD. Great job Ruthie!
About the brief writer: Lucas Tittle
Lucas Tittle is a PhD Candidate in the labs of Dr. Guillaume de Lartigue and Dr. Kevin Bolding. His research is at the intersection of the olfactory system and gut-brain axis, and how external signals like odors and internal signals interact to change feeding behavior.
If you want to read more Click here!
Nicotine and Booze: Why smoking as a teen can lead to alcohol abuse as an adult
or technically,
Adolescent nicotine exposure alters GABAA receptor signaling in the ventral tegmental area and increases adult ethanol self-administration
[See Original Abstract on Pubmed]
or technically,
Adolescent nicotine exposure alters GABAA receptor signaling in the ventral tegmental area and increases adult ethanol self-administration
[See Original Abstract on Pubmed]
Authors of the study: Alyse M. Thomas, Alexey Ostroumov, Blake A. Kimmey, Madison B. Taormina, William M. Holden, Kristen Kim, Tiffany Brown-Magnum, and John A. Dani
While human data shows a strong correlation between smoking and using drugs/alcohol, scientists have turned to rodent models to determine if nicotine is directly causing the addiction-related behaviors. Indeed, previous rodent studies have directly linked nicotine exposure to enhanced drug use. Interestingly, this association is only seen when the nicotine is administered to adolescent rodents; adult rodents exposed to nicotine do not show an increase in drug consumption later on. Given that adolescent brainsThe brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. are more flexible than fully-grown adult brainsThe brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. (in both rodents and humans!), researchers have hypothesized that nicotine exposure during adolescence may actually lead to long-term changes in brainThe brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. development. However, how these developmental changes lead to excessive drug consumption later in life was still unknown. Enter Alyse Thomas, a University of Pennsylvania researcher in the Dani lab, who was determined to find out.
While previous studies linked adolescent nicotine exposure to increased drug use, Alyse wanted to see if the same phenomenon held true when the rodents were given access to ethanol (the key ingredient in most forms of alcohol). Thinking that it would, Alyse hypothesized that adolescent (but not adult) rats exposed to nicotine would ingest excessive amounts of ethanol. To test this hypothesis, Alyse used a self-administrationa common behavioral task used by researchers to study addiction in animal models, whereby animals push a lever to give themselves a drug behavior paradigm to research the link between nicotine exposure and ethanol ingestion. This paradigm, commonly used by scientists who study drugs and addiction, allows subjects (in this case rats) to consume as much of a substance (in this case ethanol) as they desire. Alyse ran two sets of experiments: one using adolescent rats, and one using adult rats. In both experiments, the rats were injected with either nicotine or salinesalt water; a common control substance intended to have no effect on behavior or physiology for 14 days straight. Four weeks after their last injection, the rats were placed in a self-administrationa common behavioral task used by researchers to study addiction in animal models, whereby animals push a lever to give themselves a drug chamber where they were allowed unlimited access to ethanol. For the next 30 days, Alyse measured the amount of ethanol each of the rats ingested. This allowed her to determine whether short-term nicotine exposure altered ethanol consumption later in the rat’s life. As hypothesized, she found that adolescent (but not adult) rats exposed to nicotine ingested significantly more ethanol than those exposed to salinesalt water; a common control substance intended to have no effect on behavior or physiology. While this finding was expected, Alyse still wanted to know why it was the case. How does two weeks of nicotine exposure alter a rat’s behavior over four months later?
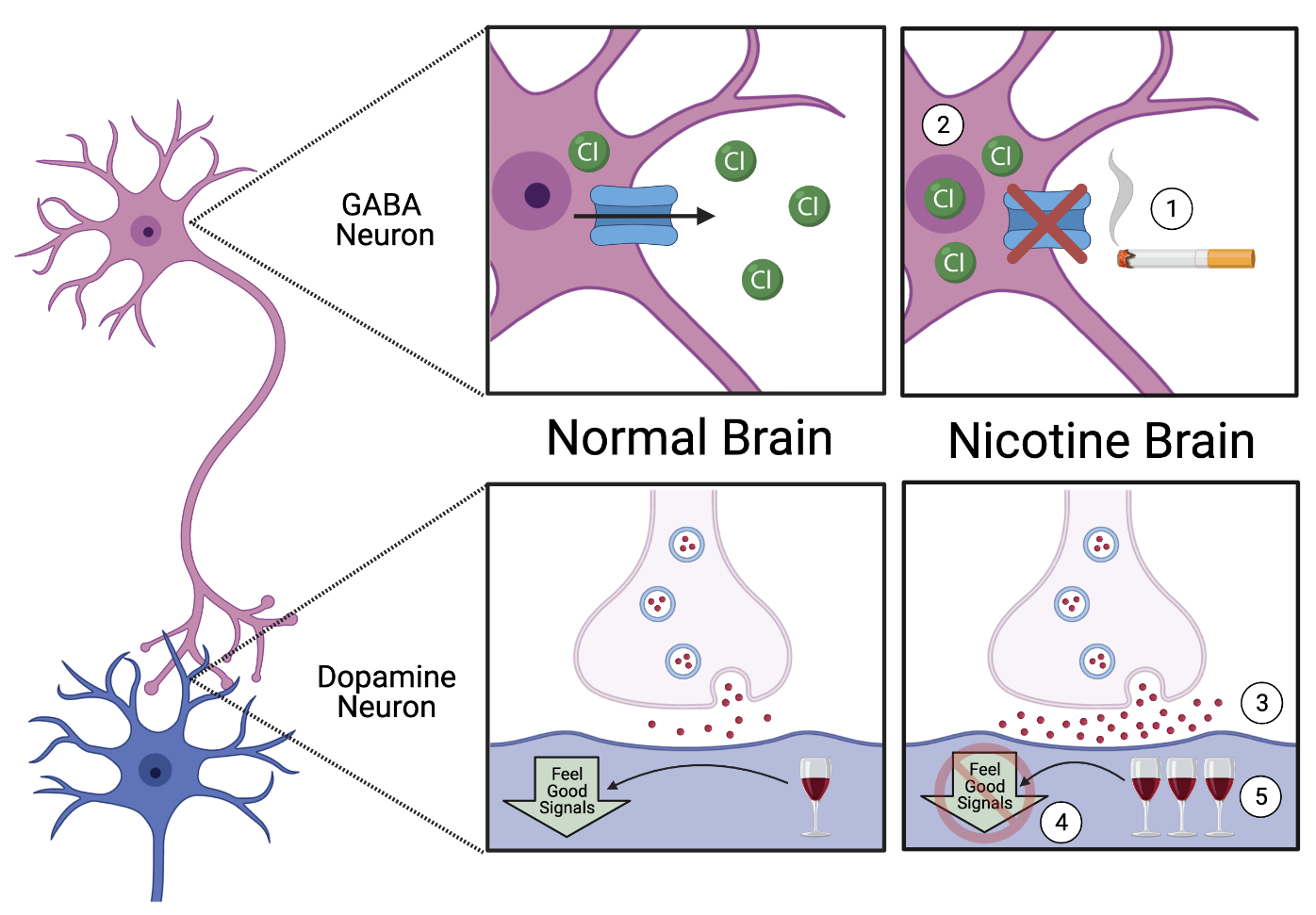
As an expert in her field, Alyse was aware that ethanol consumption is associated with changes in communication between neuronsA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles in the brainThe brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. - specifically, the neuronsA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles in the ventral tegmental areaa brain region that plays key roles in drug and natural reward response (VTA). This brainThe brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. region contains two key players: neuronsA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles that release GABA neurotransmitterschemicals released by neurons; can have an excitatory or inhibitory effect (inhibitory chemicals that “turn off” nearby neuronsA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles) and neuronsA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles that release dopamineA neurotransmitter produced by neurons in the brain that regulates movement and emotion. neurotransmitterschemicals released by neurons; can have an excitatory or inhibitory effect (excitatory, “feel good” chemicals). In the VTA, GABA neuronsA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles directly communicate with dopamineA neurotransmitter produced by neurons in the brain that regulates movement and emotion. neuronsA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles. Typically, GABA neuronsA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles release low levels of their inhibitory chemicals, causing dopamineA neurotransmitter produced by neurons in the brain that regulates movement and emotion. neuronsA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles to stay silent. However, when dopamineA neurotransmitter produced by neurons in the brain that regulates movement and emotion. neuronsA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles sense the presence of drugs or alcohol, they overpower the “off” signals sent from GABA neuronsA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles and release a flood of “feel good” signals to the rest of the brainThe brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals.. However, if the communication between these two types of neuronsA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles was impaired (say, by nicotine), the “feel good” response elicited from ethanol consumption might also be impaired. Indeed, Alyse and her labmates discovered that nicotine causes GABA neuronsA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles to become overactive, leading to underactive dopamineA neurotransmitter produced by neurons in the brain that regulates movement and emotion. neuronsA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles. Therefore, it takes more ethanol to elicit the same “feel good” response that the animal is used to, causing them to consume more.
With another piece of the puzzle in place, Alyse next wanted to know why nicotine causes these GABA neuronsA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles to become overactive. Perhaps if she could figure out the cause of the overactivation, she could figure out how to stop it. Alyse therefore took the brainsThe brain is an organ that serves as the center of the nervous system in all vertebrate and most invertebrate animals. from her adolescent nicotine- and salinesalt water; a common control substance intended to have no effect on behavior or physiology-exposed rats and looked directly into their VTA GABA neuronsA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles. Fascinatingly, she found that the nicotine-exposed neuronsA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles had a significant buildup of chloride ions within them, which could either be caused by too much chloride coming in, or not enough chloride flowing out. Alyse figured out that it was the latter scenario - nicotine-exposed GABA neuronsA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles were unable to properly expel chloride ions, causing them to build up and over activate the cell. With this last piece of the puzzle, Alyse was finally able to link adolescent nicotine exposure to increased alcohol consumption in adulthood (Figure 1).
Armed with a full understanding of the pathway, Alyse performed her final (and most important) experiment yet. Knowing that nicotine causes GABA neuronA nerve cell that uses electrical and chemical signals to send information to other cells including other neurons and muscles chloride channelsChannels are specialized proteins found on the surface of the cell. They allow molecules (i.e. neurotransmitters, salts, water) to go from outside to inside the cell and vice versa. to stop working, Alyse hypothesized that repairing these channelsChannels are specialized proteins found on the surface of the cell. They allow molecules (i.e. neurotransmitters, salts, water) to go from outside to inside the cell and vice versa. would restore the pathway and lead to a normal level of ethanol ingestion, even when adolescent rats are exposed to nicotine. Sure enough, when Alyse injected a drug that restored the chloride ion expulsion, the rats no longer ingested excessive amounts of ethanol. This finding means that not only did Alyse determine why nicotine exposure leads to excessive alcohol consumption, but she also discovered a potential treatment strategy for those suffering from alcohol addiction. As excessive alcohol consumption is responsible for more than 95,000 deaths per year in the United States alone, Alyse’s work could help reduce many tragic and preventable deaths in the future.
Figure 1. The Neurological Link Between Nicotine and Alcohol Abuse. Adolescent nicotine exposure decreases the ability of GABA neurons to get rid of chloride ions (1), causing a buildup of chloride in the cell (2). The buildup of negative charge causes the GABA neuron to release more neurotransmitter (3). As these neurotransmitters are inhibitory, they decrease the activity of the dopamine neuron that the GABA neuron is communicating with (4). Now in adulthood, the dopamine neuron requires more alcohol (5) in order to feel as “good” as the normal brain does, leading to excessive alcohol consumption.
About the brief writer: Kelsey Nemec
Kelsey is a PhD Candidate in Chris Bennett’s lab. She is interested in understanding how peripheral immune cells infiltrate the brain, with the hopes of harnessing them to treat brain diseases.